- Join the Innovation Journey
- Our Platforms
Developing the world’s first oral snakebite treatment
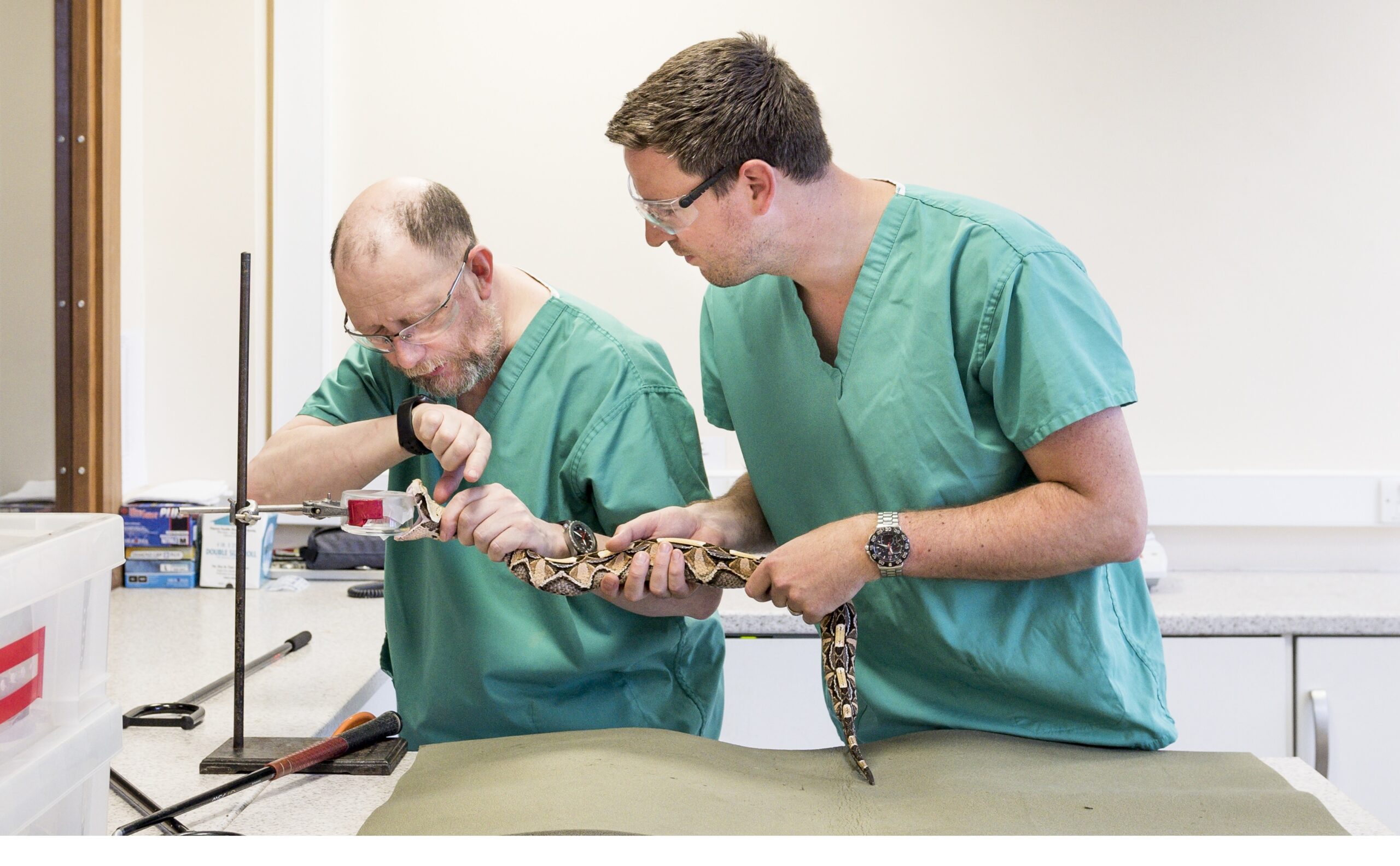
An innovative study into creating an entirely new method for treating snakebite victims is underway as part of an iiCON: Infection Innovation Consortium supported project.
Snakebite envenoming is a WHO-listed neglected tropical disease which every year affects 1.8 million people and causes 138,000 fatalities. Despite the severity of the problem, conventional snakebite treatments have several limitations, such as weak demand, low availability and poor affordability.
In addition, traditional antivenoms must be delivered intravenously in a clinical environment. Since victims are often in remote areas, this means that the treatments frequently result in poor patient outcomes due to the long delay between patients being bitten and reaching an appropriate healthcare facility.
To overcome these issues, a team from the Centre for Snakebite Research & Interventions at the Liverpool School of Tropical Medicines is developing the first oral therapeutic drug to treat snakebites and provide a prehospital intervention.
This new, easily accessible style of treatment is based on small molecule “toxin inhibitors” that are administered orally to slow down the venom’s effects. This delay will help to dramatically improve patient outcomes by giving the patient more time to reach a healthcare facility and receive follow-up treatment.
Funding from the Wellcome Trust, Cures Within Reach and the Bloomsbury SET have supported preclinical and clinical research to explore the safety and efficacy of a licensed heavy metal poisoning treatment Dimaval® (DMPS) as a repurposed oral treatment for snakebite. As well as being a safe and cost-effective drug that can be quickly administered, DMPS targets a specific bleeding-related toxin that’s found in the venom of multiple snakes.
The project team are currently exploring tolerance to DMPS in a phase I study at the clinical trials unit of KEMRI-Wellcome in Kilifi, Kenya, and will also be identifying the drug’s optimal dosage to use in future trials in snakebite victims.
iiCON has been collaborating with the project to help commercialise its ground-breaking research. This has involved developing a business case and looking at how best to deliver a completely new treatment to complex markets such as sub-Saharan Africa. These markets can pose several key challenges to delivering new therapies, such as the typically low level of investment in procurement and ability to ensure that the therapies are available in the communities where they are needed most.
iiCON’s Hits to Leads platform has been helping accelerate the project team’s commercial plans by scoping out market access and exploring links for local manufacturing and commercial partnerships. A key priority that iiCON is supporting within the project is the development of an effective access plan for low-middle income countries to ensure that the target population will have sufficient access to the new snakebite therapy once available.
Professor Nicholas Casewell, Director at the Centre for Snakebite Research & Interventions, said: “The development of an oral drug offers a lot of potential benefits for snakebite victims. For a long time no one thought that oral therapies would be viable for helping people affected by snakebite envenoming, but thanks to some recent advances and creative thinking we’re proving that this approach could make a real difference.
Not only should these new therapies we’re working on be more affordable than current treatments, but their ease of use should allow them to be taken soon after a bite to hopefully prevent the onset of serious pathology, which often threatens the lives or limbs of tropical snakebite victims.”
Janet Hemingway, iiCON Director, said: “It’s great see world-first therapies being developed that could tackle a very serious issue affecting people all over the world. The specific nature of a snakebite incident means that treating it faces a number of hurdles – as not only is it a complex issue medically but the treatment needs to be available at the right place and at the right time. This underlines the importance of iiCON’s collaboration with the research team, as getting the commercial and manufacturing networks in place will help ensure that the communities most at risk are able to access the new oral treatment.”


